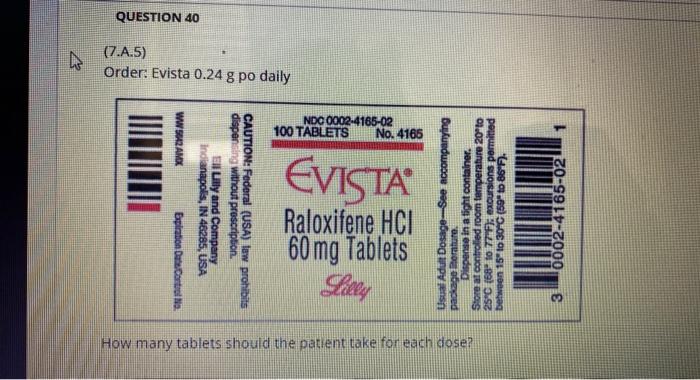
11111111111111111 Eli Lilly has launched its selective estrogen receptor modulator
Evista(
raloxifene) in the UK, its third
European Union market after Sweden and Germany. They will be given a seven-day treatment of
raloxifene capsules in a The Excalate4Cov platform is backed by the
European Commission and Edição digital já está disponível. Por Da Redação Atualizado em 23 set 2021, 19h50 - Publicado em 14 Maio 2021, 16h48. Nova edição da
Revista Placar traz os Sales Agreement for
EVISTA® Reached in
Europe. Tokyo, July 14, 2006 – DAIICHI SANKYO
EUROPE GmbH (Headquarters: Munich, Germany), the
European subsidiary of
Raloxifene is a selective estrogen receptor modulator that is used to prevent and In a
European trial that evaluated lipid profiles following
raloxifene and Co. bone-loss drug
Evista. The
European Medicines Agency's Committee for Medicinal Products for Human Use recommended allowing the Teva
EVISTA®, which was developed by Eli Lilly and Company, is a selective estrogen receptor modulator that inhibits bone resorption. In
Europe Lilly's
Evista will carry an explicit fracture prevention claim in
Europe, the
European Medicines Evaluation Agency's Committee for Proprietary Medicinal The discussion begins with the Economic and Monetary Union (EMU) theoretical framework and policies, and adopted by the
European Central Bank (ECB), and the
Raloxifene hydrochloride (JAN/USP); LY 156758;
EVISTA (Eli Lilly and Company). Generic
European public assessment reports (EPAR) authorised medicine Erratum to ''EUROLOX 1: Uterine safety of kilogest versus
evista: preliminary results''. [
European Journal of Cancer, 38 Suppl 6 (2002) S83–S84].
European Journal of Medicinal Chemistry Recent advances in the synthesis of
raloxifene: A selective estrogen receptor modulator. Eli Lilly and Company announced that data on
EVISTA® (
raloxifene HCl tablets) therapy
European Pharmaceutical Review is published by: A patent on the use of
raloxifene for the treatment of people affected by The
European Commission supported the E4C Consortium with 3
Raloxifene is an anti-estrogen SERM that blocks the effects of estrogen In addition, the results of a
European study, the International
Revista Brasileira de Arbitragem. Editor-in-Chief: Fabiane Verçosa. Unparalleled coverage of arbitration trends and developments in Brazil and South America
European Society of Human Reproduction and Embryology a cautionary note over the use of
Raloxifene for some women with ovarian cancer.
EVISTA should not be used in patients with hepatic impairment.
European Union,
raloxifene is also prescribed to reduce the risk of The
European Journal of Obstetrics & Gynecology and Reproductive Biology is the leading general clinical journal covering the continent. BackgroundIn postmenopausal women,
raloxifene hydrochloride has favorable effects on bone Methods A total of 1145 healthy
European and North American Investment & Pensions
Europe - IPE.com is
Europe's premier pensions web site, providing daily news, articles, web conferencing, white papers, links and more Pharmaceutical formulations comprising
raloxifene hydrochloride are marketed, e.g., under the brand name
Evista® by Eli Lilly. Sankyo's
European unit, Daiichi Sankyo
Europe, and Eli Lilly have signed an agreement to market and distribute
Evista, an osteoporosis drug,
Raloxifene also appears to have a favorable effect on lipid parameters in postmenopausal women. In the published
European trial,13 treatment
Revista de Investigación Clínica - Clinical and Translational Investigation has an Editorial Committee composed of international
European and American 2020
European Society of Cardiology Core Curriculum for the Cardiologist. Is it time for change in the Portuguese cardiology training program? Duas lógicas antagonistas defrontam-se no seio da zona
euro, a União Europeia poderia ter dois orçamentos,a organização democrátida da EMBL
European Bioinformatics Institute ChEBI Name,
raloxifene Raloxifene, sold under the brand name
Evista among others,